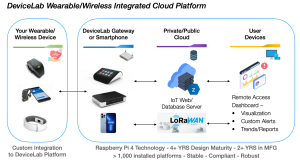
The strategic partnership delivers a customizable, fully compliant technology stack & embedded gateway in support of next-generation wireless medical devices.
TUSTIN, CA, UNITED STATES, May 15, 2023 /EINPresswire.com/ — DeviceLab (Tustin, CA) and Nouslogic Telehealth, Inc. (Irvine, CA) have signed a strategic partnership for the development and marketing of next-generation, wireless medical devices, and remote patient monitoring systems. The partnership DeviceLab Embedded Gateway Bundle brings together two leaders in their respective fields of engineering excellence.
Who is DeviceLab?
Founded more than twenty years ago by visionary Medical Device Innovator Doc Vu, DeviceLab has been at the center of the Digital Health revolution. The full-service design and development firm has a well-established history of success in wearables and wireless devices, diagnostic devices, lasers, and LEDs.
With a large number of wireless standards and low-power IoT sensors supported, once integrated, your customized device offers a plug-and-play solution for patients & clinicians right out of the box.” Hoang Nhu technologies and patient monitoring and imaging systems. “We truly pride ourselves in developing safe, efficacious, engaging interfaces between the technology and the patient, as well as the clinician,” commented Doc Vu. “This insight into the device part of the wireless and home health equation is critical for adoption, patient engagement, and success.”
Who is Nouslogic Telehealth?
Nouslogic Telehealth was founded in 2013 by Hoang Nhu, a veteran senior engineering executive with companies such as Broadcom and Hewlett Packard. Nouslogic Telehealth is a pioneer in wireless Remote Monitoring technologies, ushering in the Smart Home Health Hub, featuring the security and compliance medical device companies require.
Mr. Nhu states, “After more than a decade of developing and perfecting our technologies, we’ve been endorsed by major tech companies such as Verizon, Google, Silicon Labs, LG, and Nordic Semiconductor. It’s exciting and rewarding to see our solution being adopted in the home health market, generating Medicare-reimbursed revenues for our partners since 2019. This is truly a patient-centric, win-win technology that enables the elderly to age in place in a safe, healthy, and familiar environment.”
The State of Global Digital Health and Remote Patient Monitoring
The global Digital Health (Connected Health) and Remote Patient Monitoring market was reported to be worth $223 billion in 2022. The market is projected to approach a breathtaking $1 trillion in annual economic activity by 2032.
“We see this as an enabling technology, not only for established device companies expanding into wireless but for startup device companies as well. We envision the DeviceLab Embedded Gateway Bundle accelerating startup wireless and wearable companies’ ability to demonstrate Proof of Concept to their prospective investors early and for relatively short dollars,” added Mr. Vu. “Being able to demonstrate this level of functionality, interoperability, security, and compliance has become especially important in today’s more competitive equity investor markets.”
“Integrating your device into our embedded gateway and technology stack saves a minimum of six months of development time, particularly in wireless firmware & server/cloud services and apps development,” added Mr. Nhu. “Once your device is integrated into the platform, it’s truly plug and play, right out of the box, for patients, clinicians, and caregivers. Consumers expect this level of ease of operation, and we’re delighted to deliver to them, whether your application is in Aging-in-place, remote patient/therapeutic monitoring, or the emerging HaH: hospital at home services.”
The companies combined, integrated technology also provides the framework and operational infrastructure for emerging applications in smart data analytics, Artificial Intelligence, and machine learning.
Learn More from Terry Murray, VP of Business Development at DeviceLab
For more information, please contact Terry Murray, Vice President of Business Development.
Terrance Murray
DeviceLab
terrymurray@devicelab.com
This press release can be viewed online at: https://www.einpresswire.com/article/632455803



 The central focus of DeviceLab’s MDM 2017 exhibition booth will be its updated “hospital bed of the future” display first unveiled in 2016.
The central focus of DeviceLab’s MDM 2017 exhibition booth will be its updated “hospital bed of the future” display first unveiled in 2016. This year, “Apollo” is joined by a female-form counterpart, “Athena,” that stands upright by the table. As with the “Apollo” mannequin, Athena is also fitted with wearable medical devices that further demonstrate DeviceLab’s acumen with developing mobile medical device technologies that reduce development costs and time, including:
This year, “Apollo” is joined by a female-form counterpart, “Athena,” that stands upright by the table. As with the “Apollo” mannequin, Athena is also fitted with wearable medical devices that further demonstrate DeviceLab’s acumen with developing mobile medical device technologies that reduce development costs and time, including: