Best-in-Class Medical Device Design
DeviceLab’s Award-Winning Medical Device Team is the Best Choice for Driving Your Innovation From Concept to Commercialization
Over 20 Years of Medical Device Design Experience
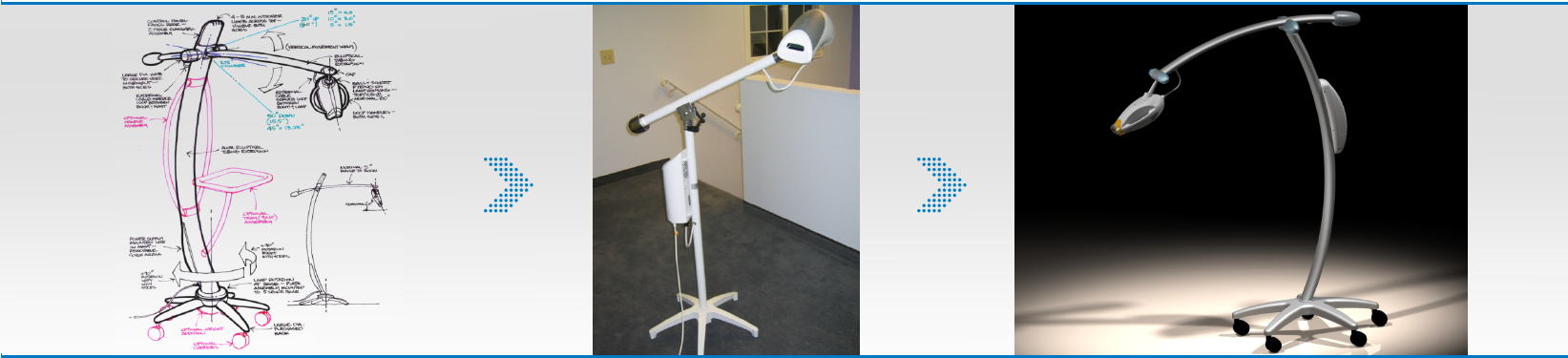
For more than 20 years, DeviceLab has been earning a well-deserved reputation for its best-in-class medical device design mastery. Our deep experience in designing award-winning medical devices is not just a reflection of our tremendously talented medical device designers in the United States, that have completed more than 300 projects of varying complexity. It also highlights our comprehensive understanding of what it truly takes to satisfy customer expectations, risk management, regulatory concerns, safety hazard requirements, standardization, and much more.


Medical Device Design for the Newest Breeds of Products and Categories
The gap between the future of medical device design and the present is rapidly closing, and nothing illustrates this more than the staggering increase of new medical devices that integrate with mobile, wireless, and IoT technologies. That is why DeviceLab launched its groundbreaking Wireless Medical Device Center in 2014.
The DeviceLab Design & Development Process
The DeviceLab medical device design process is regularly and rigorously reviewed to ensure it streamlines the process without sacrificing any critical phases or steps.
Below is an overview of our proven four-phase medical device process that ensures swift but sensible movement through the five “gates” on the path of moving from medical device concept & design to commercialization.

DeviceLab is More Than a Medical Device Design Company
We’re your champion and ally throughout a carefully designed process that recognizes your medical device project’s unique goals and objectives. As such, a medical device design project can involve any combination of the following:
- Medical Device Business Plan Analysis
- Commercialization and Ramp Up
- Concepts and Group Brainstorming
- Contract Manufacturing
- Ergonomics and Human Factors Analysis
- Feasibility Studies
- Incubation and Funding
- Legal (e.g., Intellectual property, patents, and trademarks)
- Market Research
- Marketing and Distribution
- Pilot Production and Beta Site Evaluation
- Medical Device Prototype Development
- Regulatory Compliance (e.g., navigating the Food and Drug Administration (FDA) approval process, clinical trials, acquiring CE and UL marks)
- Use, Environmental and Fatigue Testing
You Can Leverage Our Inclusion in Microchip’s 32-Bit MPU Design Partner Program
DeviceLab’s inclusion in Microchip Technology Inc.’s Design Partner Program means that your electronic medical device design project can leverage the relationship for world-class electronic medical device development.
Nearly all DeviceLab projects for medical companies include sophisticated electronic systems design and software development. As you push the boundaries of performance and power, you want your underlying design technology to be easy to use and secure, which we can reliably get from Microchip’s wide product offerings.

As part of Microchip’s 32-bit MPU specialist group, DeviceLab was specifically selected for our expertise with:
- High-speed MPU – System hardware design
- Linux software knowledge
- Secure applications
- System on Module (SoM) design capabilities
Start Getting Your Medical Device Developed Today
DeviceLab’s team makes it easy to move your new medical device from concept and design to manufacturing and commercialization. The first step is the easiest: Contact DeviceLab today to schedule a free and confidential initial medical device development consultation!